
- Applications
-
Products
-
Liquid Handling
- firefly Accelerate genomic research with innovative all-in-one, compact liquid handling
- mosquito Nanolitre liquid handling technology performs ‘traditional’ tasks at a fraction of the volume, and higher speeds
- dragonfly Delivers accurate and repeatable nanolitre to milliliter dispensing
- apricot Automated liquid handling instrumentation for convenient general use across your entire team
- Sample Preparation
-
Sample Management
- comPOUND A scalable, reliable, and secure compound management solution
- BioMicroLab Easy-to-use sample management automation instruments
- arktic Robust biospecimen storage and management down to -80°C
- lab2lab Novel sample and data transfer network system
- comPACT Reliable and efficient -20°C storage and retrieval has never been more accessible
-
Liquid Handling
-
About
- Company With a focus on liquid handling, sample preparation and sample management, our expert teams create state-of-the-art solutions that scientists and researchers can trust Culture We have one overarching mission: to work together to accelerate life science research. Through our innovative solutions and state-of-the-art tools, we believe we can make a real difference to human health Partners Collaboration is key in our mission to make a real difference to human health. Partnering with application leaders globally, we co-create to solve new challenges across the life sciences. Innovation From the initial prototype through to manufacturing, installation and beyond, we bring a problem-solving mindset and technical expertise to drive innovation
-
Executive Leadership
 Through strategic guidance, visionary thinking, and a relentless pursuit of excellence, our senior executives steer SPT Labtech towards achieving its mission of making a real difference to human health through solving advanced laboratory challenges.
Learn more
Through strategic guidance, visionary thinking, and a relentless pursuit of excellence, our senior executives steer SPT Labtech towards achieving its mission of making a real difference to human health through solving advanced laboratory challenges.
Learn more 
-
View all
 Board of Directors
Board of Directors
 Our Board of Directors are committed to driving the long-term success and sustainability of SPT Labtech, providing expert guidance and oversight to execute the company’s ambitious commercial strategy.
Learn more
Our Board of Directors are committed to driving the long-term success and sustainability of SPT Labtech, providing expert guidance and oversight to execute the company’s ambitious commercial strategy.
Learn more 
-
Knowledge Base
- Resources Our wide range of insightful resources include videos, whitepapers, eBooks, application notes and more Events & Webinars Meet the SPT team at events all over the globe and virtually via our webinars Podcast We chat with innovators and leaders from across the community to gain their unique insights. News Latest news from SPT Labtech globally Blog Our latest blog posts feature trends in research, innovative techniques and new technology
-
25 February, 2026
 SPT Labtech to collaborate with Illumina to develop automated platform supporting genomics in decentralized healthcare settings
Continue reading
SPT Labtech to collaborate with Illumina to develop automated platform supporting genomics in decentralized healthcare settings
Continue reading 
-
09 February, 2026
 SPT Labtech and BellBrook Labs Introduce High-Throughput Screening Platform for Cancer Research
Continue reading
SPT Labtech and BellBrook Labs Introduce High-Throughput Screening Platform for Cancer Research
Continue reading 
-
14 January, 2026
.jpg?length=320&name=Twist%20x%20SPT%20Labtech%20Press%20Release%20(1200%20x%20800%20px).jpg) SPT Labtech enables automated Twist Bioscience NGS library preparation workflows on firefly platform
Continue reading
SPT Labtech enables automated Twist Bioscience NGS library preparation workflows on firefly platform
Continue reading 
10
- Careers
The perfect couple for GPCR
19/07/2017






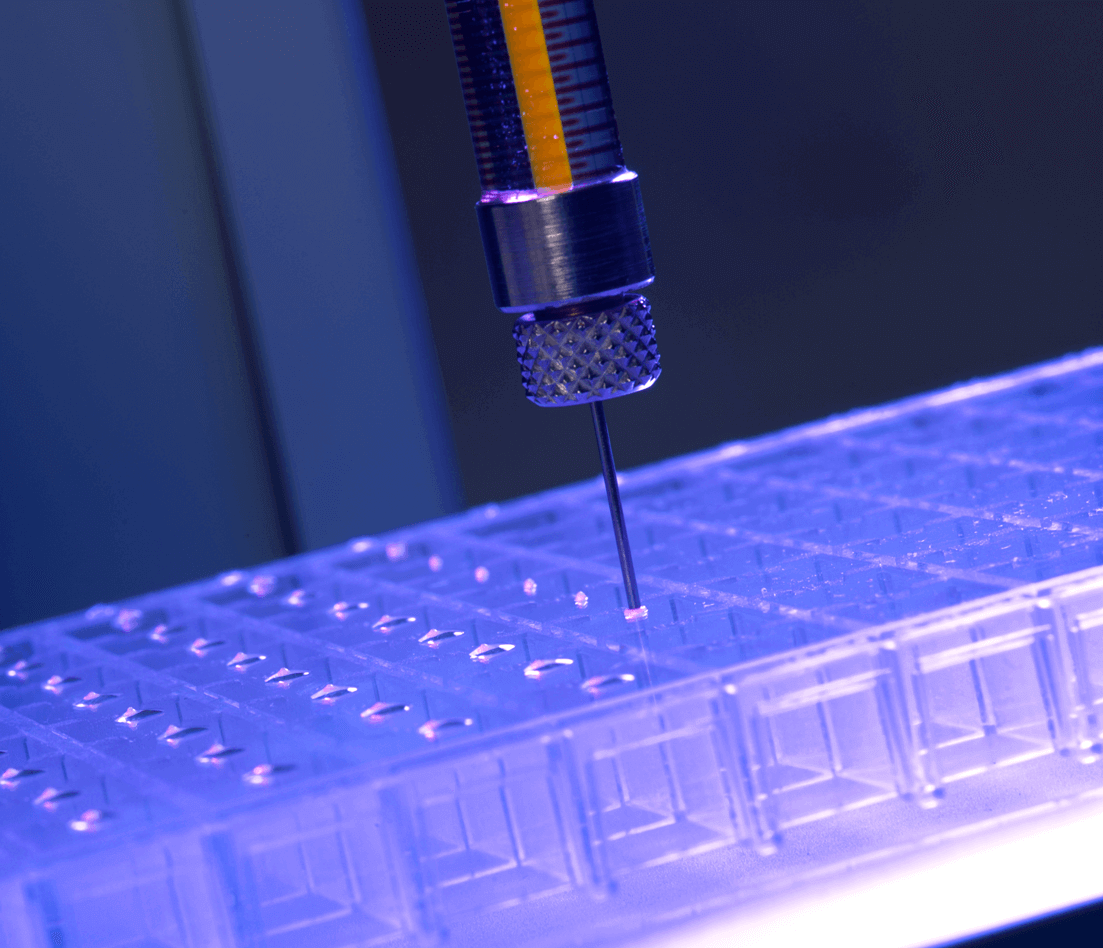 Image: LCP dispensing using mosquito® LCP
Image: LCP dispensing using mosquito® LCP