Cryo-EM has ushered in a new era of scientific discovery with the production of increasingly higher-resolution structural information. Advances in hardware and software have now opened up the technique to more widespread adoption in basic research and drug discovery. Cryo-EM is used to visualize a range of biological specimens from large cellular organelles (using cryo tomography) all the way down to the near-atomic resolution of single-particle analysis and micro-electron diffraction.

- Applications
-
Products
-
Liquid Handling
- firefly Accelerate genomic research with innovative all-in-one, compact liquid handling
- mosquito Nanolitre liquid handling technology performs ‘traditional’ tasks at a fraction of the volume, and higher speeds
- dragonfly Delivers accurate and repeatable nanolitre to milliliter dispensing
- apricot Automated liquid handling instrumentation for convenient general use across your entire team
- Sample Preparation
-
Sample Management
- comPOUND A scalable, reliable, and secure compound management solution
- BioMicroLab Easy-to-use sample management automation instruments
- arktic Robust biospecimen storage and management down to -80°C
- lab2lab Novel sample and data transfer network system
- comPACT Reliable and efficient -20°C storage and retrieval has never been more accessible
-
Liquid Handling
-
About
- Company With a focus on liquid handling, sample preparation and sample management, our expert teams create state-of-the-art solutions that scientists and researchers can trust Culture We have one overarching mission: to work together to accelerate life science research. Through our innovative solutions and state-of-the-art tools, we believe we can make a real difference to human health Partners Collaboration is key in our mission to make a real difference to human health. Partnering with application leaders globally, we co-create to solve new challenges across the life sciences. Innovation From the initial prototype through to manufacturing, installation and beyond, we bring a problem-solving mindset and technical expertise to drive innovation
-
Executive Leadership
 Through strategic guidance, visionary thinking, and a relentless pursuit of excellence, our senior executives steer SPT Labtech towards achieving its mission of making a real difference to human health through solving advanced laboratory challenges.
Learn more
Through strategic guidance, visionary thinking, and a relentless pursuit of excellence, our senior executives steer SPT Labtech towards achieving its mission of making a real difference to human health through solving advanced laboratory challenges.
Learn more 
-
View all
 Board of Directors
Board of Directors
 Our Board of Directors are committed to driving the long-term success and sustainability of SPT Labtech, providing expert guidance and oversight to execute the company’s ambitious commercial strategy.
Learn more
Our Board of Directors are committed to driving the long-term success and sustainability of SPT Labtech, providing expert guidance and oversight to execute the company’s ambitious commercial strategy.
Learn more 
-
Knowledge Base
- Resources Our wide range of insightful resources include videos, whitepapers, eBooks, application notes and more Events & Webinars Meet the SPT team at events all over the globe and virtually via our webinars Podcast We chat with innovators and leaders from across the community to gain their unique insights. News Latest news from SPT Labtech globally Blog Our latest blog posts feature trends in research, innovative techniques and new technology
-
09 February, 2026
 SPT Labtech and BellBrook Labs Introduce High-Throughput Screening Platform for Cancer Research
Continue reading
SPT Labtech and BellBrook Labs Introduce High-Throughput Screening Platform for Cancer Research
Continue reading 
-
14 January, 2026
.jpg?length=320&name=Twist%20x%20SPT%20Labtech%20Press%20Release%20(1200%20x%20800%20px).jpg) SPT Labtech enables automated Twist Bioscience NGS library preparation workflows on firefly platform
Continue reading
SPT Labtech enables automated Twist Bioscience NGS library preparation workflows on firefly platform
Continue reading 
-
11 December, 2025
 5 Key Sample Management Automation Trends for 2025- 2026: An Interview with SPT Labtech’s Cory Tiller
Continue reading
5 Key Sample Management Automation Trends for 2025- 2026: An Interview with SPT Labtech’s Cory Tiller
Continue reading 
10
- Careers
- Home
- Structural Biology
- Cryo-EM
An introduction to cryo-EM
Optimizing cryo-EM workflows improves quality and repeatability of results to advance structural biology.
The role of cryo-EM within ‘integrative’ structural biology
Cryo-EM is an important tool as part of a new era of ‘integrative’ structural biology bringing together multiple techniques to build a more comprehensive picture of dynamic processes at both a cellular and molecular level.
While the technique is increasingly adopted to tackle increasingly challenging structural biology projects, there remain opportunities to optimize workflows and overcome bottlenecks through automation.
Understanding how biomolecules function and interact is fundamental to biochemistry. This knowledge underpins fundamental research for developing new medicines and acquiring insight into the causes of infectious diseases.
Freezing samples to protect them from electron beams in Transmission Electron Microscopy (TEM) enables the study of intricate biological structures ranging from individual biomolecules to whole cells.
Frequently asked questions
There are, currently, three main cryo-EM methods commonly used in labs around the world. These are:
Single Particle Analysis
Single particle analysis (SPA) is a well-established cryo-electron microscopy technique, allowing structural biologists to investigate the detailed structures of macromolecules at a near-atomic resolution to uncover rich biological insights.
Cryo-Tomography
Cryo-electron tomography (cryo-ET) uses 3D molecular-level imaging to produce high-resolution structural and spatial information about individual proteins and the cellular environment where they operate.
Micro Electron Diffraction
Micro electron diffraction (MicroED) is a developing method for determining the structure of proteins from nanocrystals bringing together cryo-EM sample preparation approaches with electron and X-ray crystallography data analytics.
Recent technological advancements have led to significant gains in higher quality structures and cryo-EM becoming the go-to method for structural biologists. Sample preservation in vitrified ice (vitrification) is widely acknowledged as the first step in the cryogenic electron microscopy (Cryo-EM) workflow. However, before sample preparation, researchers must consider the sample quality and determine its suitability for high-resolution structure determination.
A robust understanding of these critical considerations and the correct instrumentation is vital before beginning any CryoEM project to ensure its subsequent success.
Read our blog series on getting started in sample preparation for single particle cryo-EM
Certainly, sample preparation is a recognized and as yet unresolved bottleneck in the conventional cryo-EM workflow. A standard cryo-EM workflow involves an iterative process of freezing many grids of varying quality and then screening for successful specimens using a cryogenic electron microscope. This process requires the manual handling of small, fragile grids under cryogenic conditions. The quality of resulting specimen grids is often user-dependent leading to poor consistency. Furthermore, substantial researcher experience with cryo-EM is necessary to determine ‘good’ or 'bad' sample quality as outcomes tend to be inconsistent and varied.
The main obstacle to routine structure determination of single particles by high-resolution cryo-EM remains protein adsorption to the air-water interface. It is best practice to avoid air bubbles while handling bulk protein solutions due to denaturation at the air-water interface (AWI).
In the context of thin-film formation for sample vitrification, the increased AWI surface area leads to undesirable effects such as limited particle orientation and distribution, degradation, and aggregation.
In the traditional cryo-EM sample preparation workflow, blotting is the process of removing excess aqueous sample from the grid with filter paper prior to vitrification. Inherently a difficult process to control, users must take into consideration the risks that this poses to their results.
Blotting risks sample integrity
Although commonly used, its effects on sample integrity remain to relatively be poorly defined and understood. It is difficult to know how shear forces, as well as changes to buffer concentration due to evaporation, affect individual particles. To add to the uncertainty, these effects are coupled with the often-harmful influence of the air water interface (AWI), which risks alignment of orientation, as well as particle dissociation or even denaturation.
Researchers are still trying to untangle the relationship between all these forces at play, and to better understand exactly how individual samples are adversely affected by blotting.
Blotting risks image resolution
Blotting leaves behind an uneven residual fluid layer, and therefore produces an uneven ice layer following vitrification. Ice thickness is a critical factor in image resolution, and as such, it is very difficult to reliably achieve optimal grid preparation with blotting. This oftens results in suboptimal output image resolution, wasting valuable time and resource at the microscope.
Emerging new sample preparation methods offer opportunities to drive optimization towards repeatable high-resolution outcomes on a single platform. The ability to empirically determine the sample dependent behavior for a given concentration, buffer condition, and ice thickness across a range of dispense-to-plunge times allows subsequent steps to focus on mitigating adverse effects unique to a specific sample. Taking such an approach with chameleon represents a paradigm shift in tackling sample preparation bottlenecks, ultimately reducing the downstream costs of poor sample quality.
Related Products
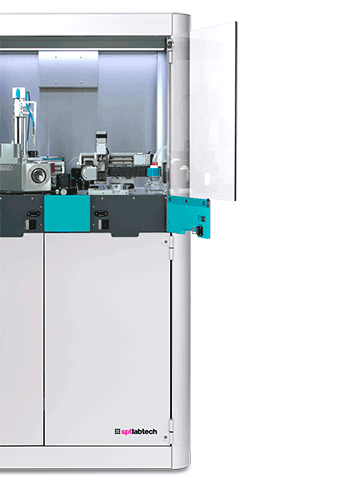
chameleon®
chameleon automates the consistent application of samples to high-quality foil grids for cryo-EM analysis, saving time and improving research outcomes across a range of projects
Explore chameleonQuantifoil
Market-leading sample supports enable cryo-EM researchers to optimize data quality.
Explore Quantifoil
As a team of exceptionally skilled scientists, engineers and business innovators, we have one mission: to work together to accelerate life science research.