Researchers need biobanks to provide high-quality samples via optimal processing, secure storage, and preservation under the appropriate conditions for the lifetime of the sample. Fundamental to the success of a biobank is the ability to preserve high-quality samples for many decades.
Automated storage solutions offer both quantitative and more intangible benefits to the biobank workflow, such as integrated sample traceability, secure storage, increased retrieval speed, and sample preservation. This article offers you a framework for justifying an automated biobank in your business plan.
Assessing your biobanking needs
Automated systems can offer high-density storage capacity, 2D-barcoded tubes for registration and tracking, plus random access and rapid cherry-picking, all of which improve the speed and organization of biobanking workflows.
A good place to start is by considering your biobank's current environment. How many samples does the facility manage? What are the processes for storage and customer order picking? What is the current electricity consumption level? For example, when managing 100,000 samples, a conventional biobank will utilize three standard -80°C freezers and manual picking. An automated biobank, however, can store this number of samples within one unit and provide effortless sample retrieval to speed up processes, while also saving energy and floor space.
By measuring and evaluating costs relative to financial savings from a possible automated solution, an investment analysis can give a clear idea of the business case for investing in an automated biobank. The option to install a modular storage solution rather than a single large store can offer flexible financial benefits over the working life of the system.
The total cost of sample management can be attributed to equipment and service, labor, electricity, and other intangibles. While this latter category is not always easy to quantify, it is important to include because the intangible costs can severely impact the biobank's reputation for quality and performance.
Tangible costs:
- Labor costs
- Energy costs
- Space
Intangible costs:
- Sample integrity
- Sample accessibility
- Data integrity/security
With funding circumstances constantly evolving, capital investment costs versus anticipated returns are integral to analysis when considering automation. Building an operational cost model for the working life of a system that considers library size, throughput requirements and the value placed on the intangible benefits of automation can help to determine whether full or partial automation is the right move. Predicting future usage can be difficult so consider investing in scalable solutions that grow with the biobank to soften initial investment costs. The alternative approach requires investing in a large biobanking option with unused space set aside for potential growth.
Considering that services account for an estimated 10% of the cost of a storage system, premature planning for future growth not only increases the initial investment costs but also associated service costs.
One further consideration lies in the implementation time frames and knock-on delays to downstream workflows when installing automated solutions. A typical time frame for implementing a large automated system can be two to three years, however, smaller modular stores such as those from SPT Labtech can be up and running in as little as a week.
Finally, it's important to weigh up the facility's overall comfort level with new technologies to help justify the jump to automation and consider including integration with LIMS, particularly if in a technology-driven environment.
An automation cost analysis
In order to compare the differences between manual and automated sample retrieval approaches, a study was conducted by SPT Labtech to establish the average picking time at a UK biobank where samples were stored in manual freezers. This study revealed that the store was already 10% fragmented, i.e. had 10% empty space within the storage racks from previously retrieved samples.
The study also found that a picking session typically took 10 minutes of preparation time. Individual samples were picked on average every 26 seconds with a further 10 seconds required per sample for database management.
The following compares the total costs of using a manual freezer compared to SPT Labtech's arktic® -80°C automated freezer when picking 100 (1 rack), 500 (5 racks) or 1000 (10 racks) samples per week, based on a 48-week year.
100,000 samples stored in one SPT Labtech’s arktic -80°C automated freezer equates to an overall cost of approximately €675,000 spread over a 10-year period. This includes the initial cost of the system, service (10% of the system cost annually) and electricity. Electricity costs can be grouped into the overall cost of this solution with confidence because air exchange is minimal when picking samples, i.e. there is no difference in actively picking and managing samples versus passive freezing.
100,000 vials (0.5ml) fit in an arktic, but three manual freezers would be needed to hold the same number. The resulting manual storage costs over 10 years include the initial outlay, plus service costs associated with three freezers at €90,000. At first glance this seems the better option, but one must not forget to include library management and sample picking labor costs as well as fluctuating electricity costs that have now become much more significant factor.
For example, SPT Labtech measured the power usage during a one hour picking session, finding that manual freezers averaged 1.7 kW for three hours, followed by cooling for two hours at 1.4 kW until reaching the goal temperature of -80°C where passive energy consumption was measured at 1.1 kW. Given the manual nature of sample picking where freezers are repeatedly opened and closed, power usage increases each time a picking session occurs. As such, a more accurate cost comparison should include an accurate measure of energy usage and electricity costs for the two approaches.
Two cost models
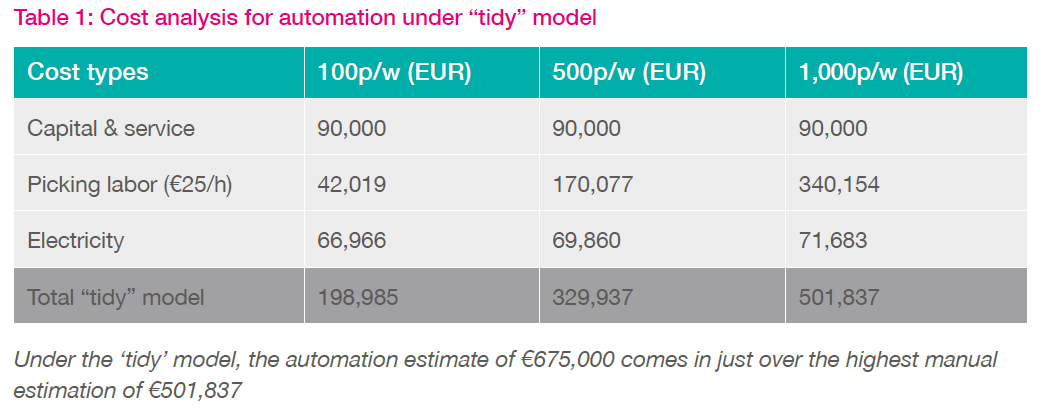
Based upon observed timings from the aforementioned study at a UK biobank, the cost analysis outlined in Table 1 below explores two model scenarios. The first model assumes a very 'tidy' process with no manual error or defragmentation. Here the average pick time of 26 seconds was doubled to account for the additional time required to keep the store organized (i.e. the re-filling of racks post-retrieval) and freezers operating at maximum capacity. The data in Table 1 demonstrate that picking more samples per week (p/w) leads to a significant increase in labor costs and a slight increase in electricity costs.
Please note that the costs used here are based on 2019 data, so would be higher today, especially when considering the rate of inflation and recent significant rises in energy costs.
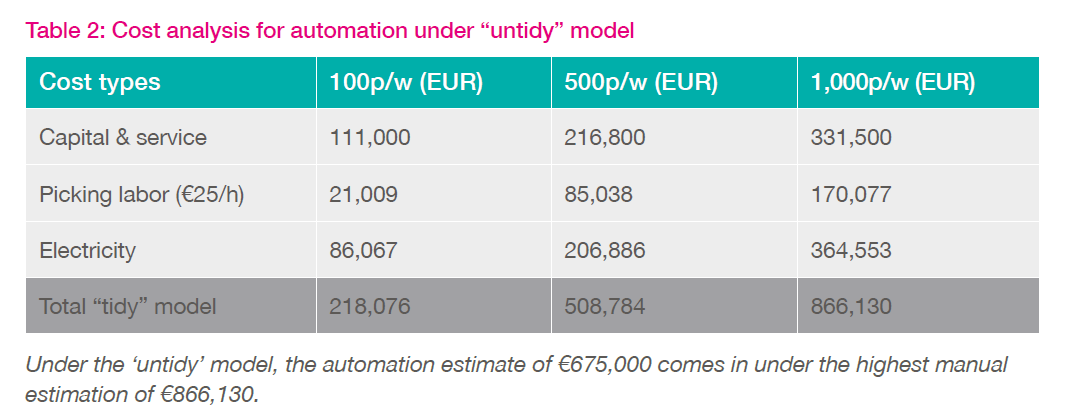
The second model (Table 2) assumes a more inefficient process where racks were not refilled after sample picking. This approach quickly leads to a fragmented and 'untidy', biobank. In this second model, all category costs escalate for the busier (500-1000 p/w) biobanks due to a) the need for additional freezers (b) extended time spent by staff looking for samples, c) more electricity used. While these represent two extreme cases, the average biobank lies somewhere in between, with occasional defragmentation required to create more capacity, some wasted space and more freezers than necessary.
Based solely on this cost-based analysis, biobank automation is clearly justified when picking 500-1,000+ samples per week. For lower pick rates, one should most certainly consider the level of priority that the more intangible factors of automation might have. These include space savings, workflow efficiency, sample accessibility, integrity, and security. While a cost analysis of these factors is more difficult to quantify, they represent extremely important decision-making criteria. When it comes to improved sample integrity and data quality, what additional cost is acceptable?
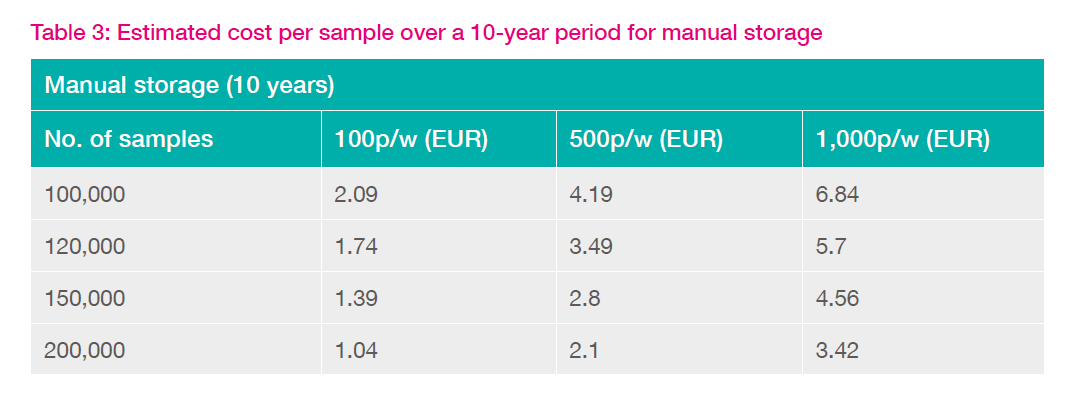
To assist in the justification process, a perspective on the cost per sample for automated and manual approaches is useful. Tables 2 and 3 demonstrate the effect of library size (i.e. the number of samples stored) on cost per sample when stored manually or in an automated system.
Table 3 also clearly demonstrates that for pick rates of over 1,000 samples per week automation is clearly justified for all library sizes. For lower pick rates (100-500 p/w), there is a supplementary cost over and above that of manual storage.
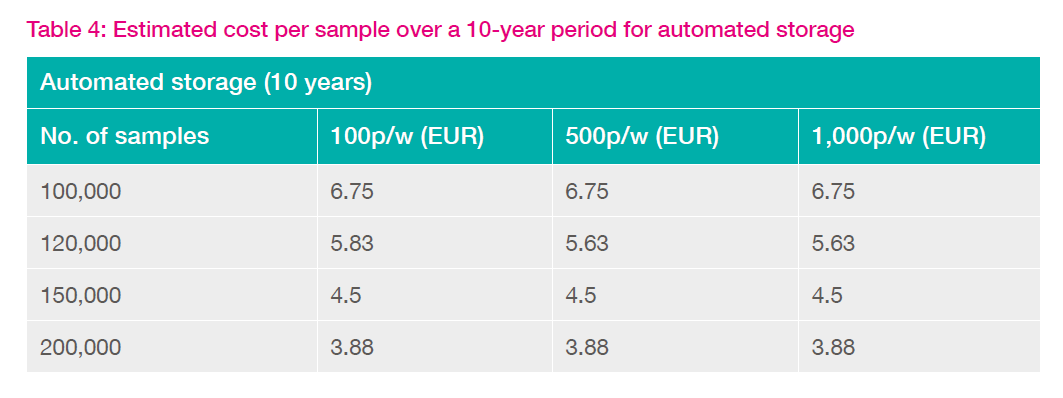
The cost per sample difference between manual and automated solutions is reported in Table 4. Note that calculations cover a 10-year period to represent the approximate lifespan of the storage solution.
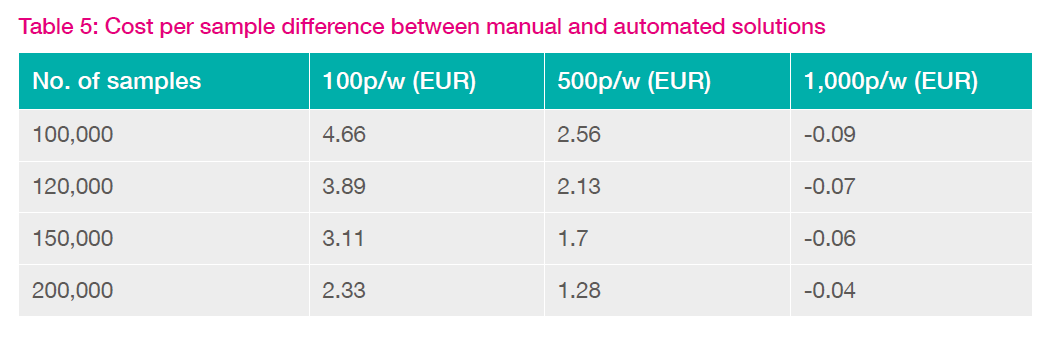
Accounting for sample integrity – the 'penguin effect'
One of the most important factors for biobanks to consider is sample integrity. When it comes to the protection of innocent samples, can the price differential between manual and automated solutions highlighted in Table 4 be justified?
Reduced sample integrity can potentially result in the loss of precious data points, or unreliable data. To investigate the latter, SPT Labtech studied the sample freezing and sample picking profiles of 1.4 mL vials each containing 0.97 mL in manual freezers, across different locations. These tests evaluated temperature variability in rack-based storage systems common in standard freezers and some automated solutions.
When freezing samples in a -80°C freezer, very different freezing profiles were observed depending on vial location in the racks and rack location in the freezer. A 'penguin effect' was described where outer samples freeze within five to twenty minutes while inner samples take over one hour. The test revealed a seventy-minute difference between outer and inner samples reaching -40°C, highlighting a significant insulation effect for inner vials.
When picking samples from a -80°C freezer, the penguin effect is observed in reverse when placing samples on dry ice. Here, outer vials warm faster, with up to a 50°C temperature change in less than ten minutes. Thus, there is a false sense that using dry ice can maintain the sample temperature at a steady state.
Furthermore, the repeated opening and closing of the freezer door during picking can do more harm than one would expect to the innocent samples that remain in the freezer. The monitored of temperature of samples in a freezer during one hour of picking were warmer than -70°C, with samples nearest to the door reaching -55°C. Note, this test lasted only one hour so the maximum temperature reached before cooling resumed was not recorded.
By comparison, SPT Labtech’s automated storage systems demonstrate great care and attention to sample integrity through rack-less storage. Here the 'penguin' effects observed in both manual freezers and other automated storage systems are eliminated. Each sample tube is stored in an individual storage location with consistent and uniform temperature profiles to ensure each tube experiences the same freezing profile. By maintaining a closed air exchange, temperature fluctuations when cherry picking individual samples are minimized versus those experienced by repeatedly opening and closing a freezer door, thus protecting innocent samples.
An additional benefit of this approach is that each storage location can be accessed directly. Following a sample’s retrieval, its location becomes immediately available for the subsequent storage of another sample. The fragmentation issues associated with manual and automated systems utilizing racks for storage are therefore circumvented, freeing up valuable time and resource.
Based on this cost analysis and considering those less quantifiable factors, a biobank can conclude that 1,000 picks per week justifies automation purely based on cost. 500 or more picks per week can be arguably justified by balancing cost with other factors. When picking less than 500 samples per week, a cost-based justification for automation is not obvious. However, where factors such as sample integrity are weighted highly, automation provides significant quality assurances.
Comparing automated storage options
There are two automated storage options worth comparing. Many biobanks looking to adopt automated storage predict their capacity needs for up to 10 years. Therefore, the first approach is to install a system large enough to meet those 10-year projections.
A second approach is to install a modular solution, such as SPT Labtech's arktic XC, which allows for the system to grow in line with needs, making it easier to adapt to evolving and/or unforeseen needs.

To explore the benefits of this second approach, consider a biobank storing 100,000 samples per year for 10 years.
Cost per sample for a large store (Option 1)
Note that the total cost includes purchase cost, ongoing service costs and electricity costs, but not any required infrastructure changes which can be considerable.
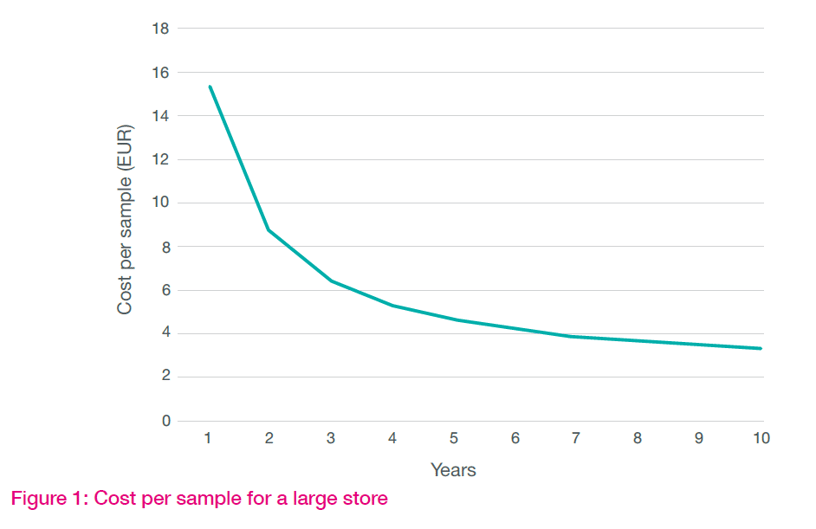
Cost per sample illustration for a large automated store growing at 100,000 samples per year to a final capacity of 1M samples
The cost per sample for Option 1 ranges from approx. €15 initially to €3.2 by the time the store is full. After 10 years the store may be reaching the end of its working life and will require new funding to replace and/or expand the store to provide for further growth. Option 1 is therefore inflexible to unforeseen changes that may happen in the future.
Cost per sample for a modular system (Option 2)
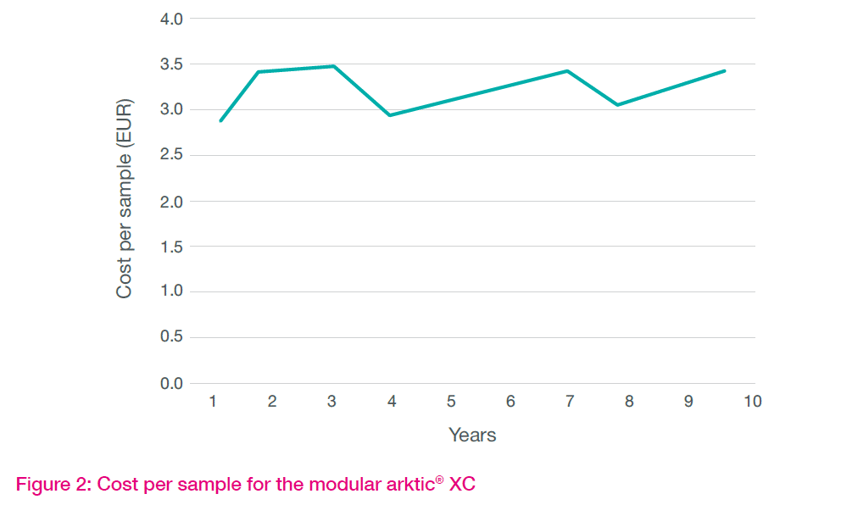
As in Option 1, the total cost for Option 2 includes purchase, service, and electricity. But, in the case of a modular system, infrastructure changes are generally not needed due to the compact module size. The cost per sample ranges from €3.4 to €2.8. In other words, it only fluctuating a little with the addition of integrated modules when required. Option 2 is highly adaptable to future changes with limitless expansion possibilities.
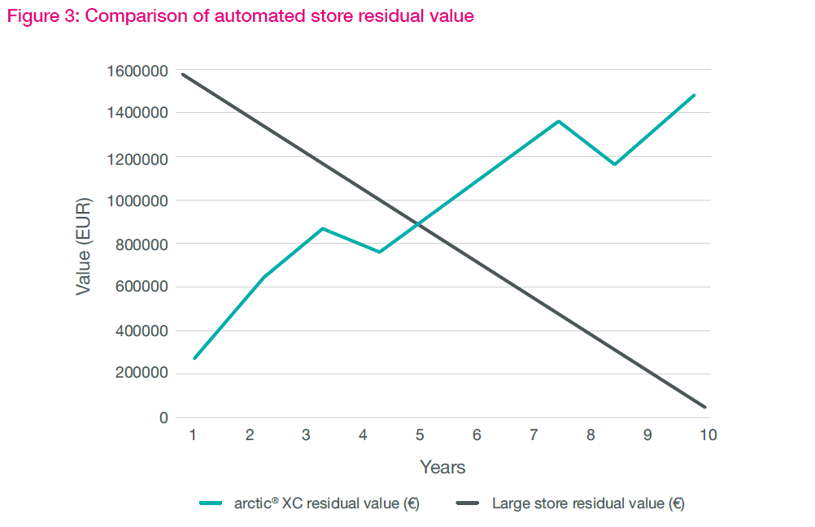
Depreciation of assets
Considering the residual asset values over the 10-year period is also quite revealing. The arktic XC approach enables a rolling program of module replacement, compared to the need to install a whole new system.
Need more detailed costings?
If you would like to discuss the above in more detail and in relation to your own biostorage requirements, please reach out to us. Our biobanking experts would love to hear from you!


 Through strategic guidance, visionary thinking, and a relentless pursuit of excellence, our senior executives steer SPT Labtech towards achieving its mission of making a real difference to human health through solving advanced laboratory challenges.
Learn more
Through strategic guidance, visionary thinking, and a relentless pursuit of excellence, our senior executives steer SPT Labtech towards achieving its mission of making a real difference to human health through solving advanced laboratory challenges.
Learn more 
 Board of Directors
Board of Directors
 Our Board of Directors are committed to driving the long-term success and sustainability of SPT Labtech, providing expert guidance and oversight to execute the company’s ambitious commercial strategy.
Learn more
Our Board of Directors are committed to driving the long-term success and sustainability of SPT Labtech, providing expert guidance and oversight to execute the company’s ambitious commercial strategy.
Learn more 
 SPT Labtech and BellBrook Labs Introduce High-Throughput Screening Platform for Cancer Research
Continue reading
SPT Labtech and BellBrook Labs Introduce High-Throughput Screening Platform for Cancer Research
Continue reading 
.jpg?length=320&name=Twist%20x%20SPT%20Labtech%20Press%20Release%20(1200%20x%20800%20px).jpg) SPT Labtech enables automated Twist Bioscience NGS library preparation workflows on firefly platform
Continue reading
SPT Labtech enables automated Twist Bioscience NGS library preparation workflows on firefly platform
Continue reading 
 5 Key Sample Management Automation Trends for 2025- 2026: An Interview with SPT Labtech’s Cory Tiller
Continue reading
5 Key Sample Management Automation Trends for 2025- 2026: An Interview with SPT Labtech’s Cory Tiller
Continue reading 















